Unlocking the Power of Hydrogen: A Low-Carbon Future
Hydrogen has the potential to become a cornerstone of a sustainable energy system, especially as countries seek cleaner energy solutions to mitigate climate change.
As the International Energy Agency (IEA) estimates, hydrogen could account for 18% of global energy demand by 2050, presenting a significant opportunity to decarbonise various industries.
Yet, concerns about the environmental impact of hydrogen production, transportation, and use remain, particularly regarding how different hydrogen production methods contribute to greenhouse gas emissions.
As the International Energy Agency (IEA) estimates, hydrogen could account for 18% of global energy demand by 2050, presenting a significant opportunity to decarbonise various industries.
Yet, concerns about the environmental impact of hydrogen production, transportation, and use remain, particularly regarding how different hydrogen production methods contribute to greenhouse gas emissions.
Green Hydrogen Production: A Sustainable Path to a Low-Carbon Future
Hydrogen production is a critical component of the hydrogen economy and has significant environmental implications. In this chapter, we will examine the different methods of hydrogen production, their ecological effects, and the opportunities and challenges associated with each method.
Methods of Hydrogen Production
At Hydrogenera, we produce green hydrogen through alkaline electrolysis, which involves using electricity to split water into hydrogen and oxygen. This method is powered by renewable energy sources, making it a promising low-carbon hydrogen production method.
Other methods of hydrogen production include:
Why Electrolysis?
We have chosen to focus on electrolysis as our method of hydrogen production because it offers several advantages. First, it produces no greenhouse gas emissions if powered by renewable energy sources. Second, it is a highly efficient process that can be powered by excess energy from renewable sources. Finally, it produces high-purity hydrogen that can be used directly in fuel cells, Internal Combustion Engines, or other applications.
Environmental Effects of Hydrogen Production
The environmental effects of hydrogen production vary depending on the method used.
Here is a comparison of the environmental effects of different hydrogen production methods:
For a more in-depth look at the different hydrogen colours and their implications, please refer to our previous article: “What Do Hydrogen Colors Mean?”
Methods of Hydrogen Production
At Hydrogenera, we produce green hydrogen through alkaline electrolysis, which involves using electricity to split water into hydrogen and oxygen. This method is powered by renewable energy sources, making it a promising low-carbon hydrogen production method.
Other methods of hydrogen production include:
- Steam Methane Reforming (SMR): This is the most common method of hydrogen production, accounting for approximately 95% of global hydrogen production. SMR involves the reaction of high-temperature steam with methane to produce hydrogen and carbon monoxide.
- Coal Gasification: This method involves the reaction of coal with high-temperature steam to produce hydrogen and carbon monoxide.
- Biological Production: This method involves using microorganisms to produce hydrogen through fermentation or photosynthesis.
- Partial oxidation is a common method for generating hydrogen. In this process, a hydrocarbon fuel, often natural gas or liquid hydrocarbons, reacts with limited oxygen, producing hydrogen gas, carbon monoxide, and carbon dioxide.
- Pyrolysis is an advanced hydrogen production method where methane is broken down into hydrogen and solid carbon at high temperatures without oxygen. Unlike other processes, it produces solid carbon instead of CO₂, making it easier to manage. When using biomethane or biomass, pyrolysis can create “super green” hydrogen, offering a negative-emission solution by trapping carbon and reducing greenhouse gas emissions.
Why Electrolysis?
We have chosen to focus on electrolysis as our method of hydrogen production because it offers several advantages. First, it produces no greenhouse gas emissions if powered by renewable energy sources. Second, it is a highly efficient process that can be powered by excess energy from renewable sources. Finally, it produces high-purity hydrogen that can be used directly in fuel cells, Internal Combustion Engines, or other applications.
Environmental Effects of Hydrogen Production
The environmental effects of hydrogen production vary depending on the method used.
Here is a comparison of the environmental effects of different hydrogen production methods:
- Electrolysis: This method produces no greenhouse gas emissions if powered by renewable energy.
- Grey Hydrogen (Steam Methane Reforming - SMR): Grey hydrogen, produced from natural gas, accounts for 95% of global hydrogen production. While more carbon-efficient than coal, it still releases significant greenhouse gases.
- Blue Hydrogen (SMR with Carbon Capture): While slightly better than grey hydrogen, blue hydrogen still relies on fossil fuels, and carbon capture is not 100% efficient. Studies have shown that even blue hydrogen can emit up to 20% of the CO₂ produced in grey hydrogen processes.
For a more in-depth look at the different hydrogen colours and their implications, please refer to our previous article: “What Do Hydrogen Colors Mean?”
Getting Hydrogen to Where It's Needed: Safe and Efficient Transportation and Storage
Once hydrogen is produced, it must be transported to the point of use, which can be a significant challenge. This chapter will examine the different hydrogen transportation and storage methods, their advantages and disadvantages, and the critical considerations for safe and efficient transportation.
Methods of Hydrogen Transportation
There are several methods of hydrogen transportation, including:
Methods of Hydrogen Storage
Hydrogen can be stored in several forms, including:
Critical Considerations for Safe and Efficient Transportation
The essential considerations for the safe and efficient transportation of hydrogen include the following:
In conclusion, the transportation and storage of hydrogen are critical components of the hydrogen economy.
By understanding the different methods of transportation and storage and the critical considerations for safe and efficient transportation, we can ensure that hydrogen is delivered to the point of use safely and efficiently.
Methods of Hydrogen Transportation
There are several methods of hydrogen transportation, including:
- Pipelines: Hydrogen can be transported through pipelines, typically used for high-volume transportation over long distances.
- Trucks: Hydrogen can be transported by truck, typically for smaller volumes and shorter distances.
- Trains: Hydrogen can be transported by train and is typically used for larger volumes and longer distances.
- Ships: Hydrogen can be transported by ship and is typically used internationally.
Methods of Hydrogen Storage
Hydrogen can be stored in several forms, including:
- Compressed Hydrogen is the most well-known method of hydrogen storage. It involves compressing hydrogen gas under high pressure, typically up to 700 bar or more, in specialised tanks. It is a relatively simple and widely used technology for fuel cell vehicles, especially in the automotive industry. However, compressing hydrogen requires a significant amount of energy.
- Liquefied Hydrogen: Storing hydrogen as a liquid requires cryogenic temperatures, as its boiling point at one atmosphere is −252.8°C. This method offers a much higher energy density than compressed hydrogen, making it suitable for larger volumes and longer-term storage. However, the liquefaction process is energy-intensive, and effective thermal insulation of the storage vessel is crucial to minimise hydrogen boil-off.
- Cryo-compressed Hydrogen Storage: Cryo-compressed hydrogen is stored as a supercritical cryogenic gas, where hydrogen remains gaseous but is compressed at about -233°C. The storage vessels are designed to handle the internal pressure from the cryogenic gas, featuring a vacuum enclosure for enhanced safety and insulation. This method provides high storage density, efficient refuelling, and avoids hydrogen boil-off, as the tanks are built to operate under high pressure.
- Liquid Organic Hydrogen Carrier (LOHC) is a way to store hydrogen using liquid organic compounds. These liquids can absorb and store hydrogen, making them convenient and safe to transport and store. The process works like this: the liquid compound reacts with hydrogen to form a hydrogen-rich liquid. This liquid can be stored or transported at average temperatures and pressures, making it a practical solution. The liquid can be easily converted into hydrogen gas when the hydrogen is needed.
Critical Considerations for Safe and Efficient Transportation
The essential considerations for the safe and efficient transportation of hydrogen include the following:
- Pipeline safety: Hydrogen pipelines must be designed and constructed to withstand the high pressures and temperatures associated with hydrogen transportation.
- Leak detection: Hydrogen leaks can be challenging, so advanced leak detection systems are essential for safe transportation.
- Compressor station safety: Compressor stations must be designed and constructed to withstand the high pressures and temperatures associated with hydrogen compression.
In conclusion, the transportation and storage of hydrogen are critical components of the hydrogen economy.
By understanding the different methods of transportation and storage and the critical considerations for safe and efficient transportation, we can ensure that hydrogen is delivered to the point of use safely and efficiently.
Unlocking the Potential of Hydrogen Utilization: Opportunities and Applications
Hydrogen has various applications across various industries, and its potential still needs to be explored. This chapter will explore how hydrogen can be utilised and the opportunities and challenges associated with each application.
Transportation
Hydrogen is a clean and efficient fuel for transportation with several applications:
There are several examples worldwide of successful implementation of such processes.
Norway is expected to play a significant role in meeting the EU's climate goals. The country has launched several green and blue hydrogen projects, with Enova and the EU funding, to develop infrastructure for clean energy production and transportation.
Power Generation
Hydrogen can be used to generate electricity in a variety of applications, including:
Industrial Processes
Hydrogen is used in a variety of industrial processes, including:
Transportation
Hydrogen is a clean and efficient fuel for transportation with several applications:
- Fuel Cell Electric Vehicles (FCEVs) offer a zero-emission alternative to traditional gasoline-powered vehicles.
- Internal Combustion Engines (ICE) can also operate on hydrogen, providing a cost-effective and well-established alternative to traditional gasoline-powered engines. Hybrid vehicles combine a hydrogen fuel cell with a battery or traditional engine, offering improved fuel efficiency and reduced emissions.
- Hydrogen can also power ships and boats, offering a cleaner alternative to fossil fuels.
There are several examples worldwide of successful implementation of such processes.
Norway is expected to play a significant role in meeting the EU's climate goals. The country has launched several green and blue hydrogen projects, with Enova and the EU funding, to develop infrastructure for clean energy production and transportation.
Power Generation
Hydrogen can be used to generate electricity in a variety of applications, including:
- Gas turbines generate electricity by burning hydrogen in a combustion chamber.
- Fuel cells generate electricity through a chemical reaction between hydrogen and oxygen.
- Combined heat and power (CHP) systems generate electricity and heat from a single energy source.
Industrial Processes
Hydrogen is used in a variety of industrial processes, including:
- Chemical synthesis is where hydrogen is used to synthesise chemicals such as ammonia and methanol.
- Refining and processing: Hydrogen is used to refine and process fossil fuels, such as petroleum.
- Metallurgy is where hydrogen extracts and processes metals, particularly in reducing and purifying iron ore for steel production.
The Environmental Impacts of Hydrogen Production, Transportation, and Use
The environmental impacts of hydrogen production, transportation, and use vary significantly depending on the hydrogen produced.
Green Hydrogen: A Minimal Environmental Footprint
Green hydrogen, also known as renewable hydrogen, is produced from renewable energy sources such as solar or wind power. This type of hydrogen production has a minimal environmental footprint, as it generates no greenhouse gas emissions during production. The only by-products are water vapour and heat.
Green hydrogen transportation is also environmentally friendly, as it can be transported through natural gas pipelines with minimal modifications. Additionally, using green hydrogen in fuel cell vehicles or power generation has zero tailpipe emissions, reducing air pollution and greenhouse gas emissions.
Grey Hydrogen: A Moderate Environmental Impact
Grey hydrogen, on the other hand, is produced from fossil fuels such as natural gas. While it is still a cleaner energy source than traditional fossil fuels, grey hydrogen production generates moderate greenhouse gas emissions during the extraction and processing of natural gas.
Using grey hydrogen in fuel cell vehicles or power generation also generates some greenhouse gas emissions, although significantly less than traditional fossil fuels.
Blue Hydrogen: A Moderate Environmental Impact
Blue hydrogen is produced from natural gas using Steam Methane Reforming (SMR). While this process reduces the carbon intensity of hydrogen production, it still generates significant greenhouse gas emissions during the extraction and processing of natural gas.
Comparison with Traditional Fossil Fuels
Green hydrogen is a game-changer for the environment compared to traditional fossil fuels. While grey and blue hydrogen have some environmental impacts, they are still cleaner energy sources than conventional fossil fuels.
However, the production and use of grey and blue hydrogen generate significant greenhouse gas emissions, contributing to climate change.
The transportation of traditional fossil fuels also has devastating environmental effects, including air pollution and oil spills.
In conclusion, green hydrogen has a minimal environmental impact compared to other hydrogen or traditional fossil fuels. Its production, transportation, and use produce zero greenhouse gas emissions, making it an attractive alternative for a sustainable future.
While grey and blue hydrogen have some environmental impacts, they are still cleaner energy sources than traditional fossil fuels. However, the devastating ecological effects of polluting carbon emissions from conventional fuels make them a less desirable option for our planet's well-being.
For example, a Hydrogenera 1MW electrolyser produces 423 Kilograms of hydrogen in 24 hours. This is equal to:
Green Hydrogen: A Minimal Environmental Footprint
Green hydrogen, also known as renewable hydrogen, is produced from renewable energy sources such as solar or wind power. This type of hydrogen production has a minimal environmental footprint, as it generates no greenhouse gas emissions during production. The only by-products are water vapour and heat.
Green hydrogen transportation is also environmentally friendly, as it can be transported through natural gas pipelines with minimal modifications. Additionally, using green hydrogen in fuel cell vehicles or power generation has zero tailpipe emissions, reducing air pollution and greenhouse gas emissions.
Grey Hydrogen: A Moderate Environmental Impact
Grey hydrogen, on the other hand, is produced from fossil fuels such as natural gas. While it is still a cleaner energy source than traditional fossil fuels, grey hydrogen production generates moderate greenhouse gas emissions during the extraction and processing of natural gas.
Using grey hydrogen in fuel cell vehicles or power generation also generates some greenhouse gas emissions, although significantly less than traditional fossil fuels.
Blue Hydrogen: A Moderate Environmental Impact
Blue hydrogen is produced from natural gas using Steam Methane Reforming (SMR). While this process reduces the carbon intensity of hydrogen production, it still generates significant greenhouse gas emissions during the extraction and processing of natural gas.
Comparison with Traditional Fossil Fuels
Green hydrogen is a game-changer for the environment compared to traditional fossil fuels. While grey and blue hydrogen have some environmental impacts, they are still cleaner energy sources than conventional fossil fuels.
However, the production and use of grey and blue hydrogen generate significant greenhouse gas emissions, contributing to climate change.
The transportation of traditional fossil fuels also has devastating environmental effects, including air pollution and oil spills.
In conclusion, green hydrogen has a minimal environmental impact compared to other hydrogen or traditional fossil fuels. Its production, transportation, and use produce zero greenhouse gas emissions, making it an attractive alternative for a sustainable future.
While grey and blue hydrogen have some environmental impacts, they are still cleaner energy sources than traditional fossil fuels. However, the devastating ecological effects of polluting carbon emissions from conventional fuels make them a less desirable option for our planet's well-being.
For example, a Hydrogenera 1MW electrolyser produces 423 Kilograms of hydrogen in 24 hours. This is equal to:
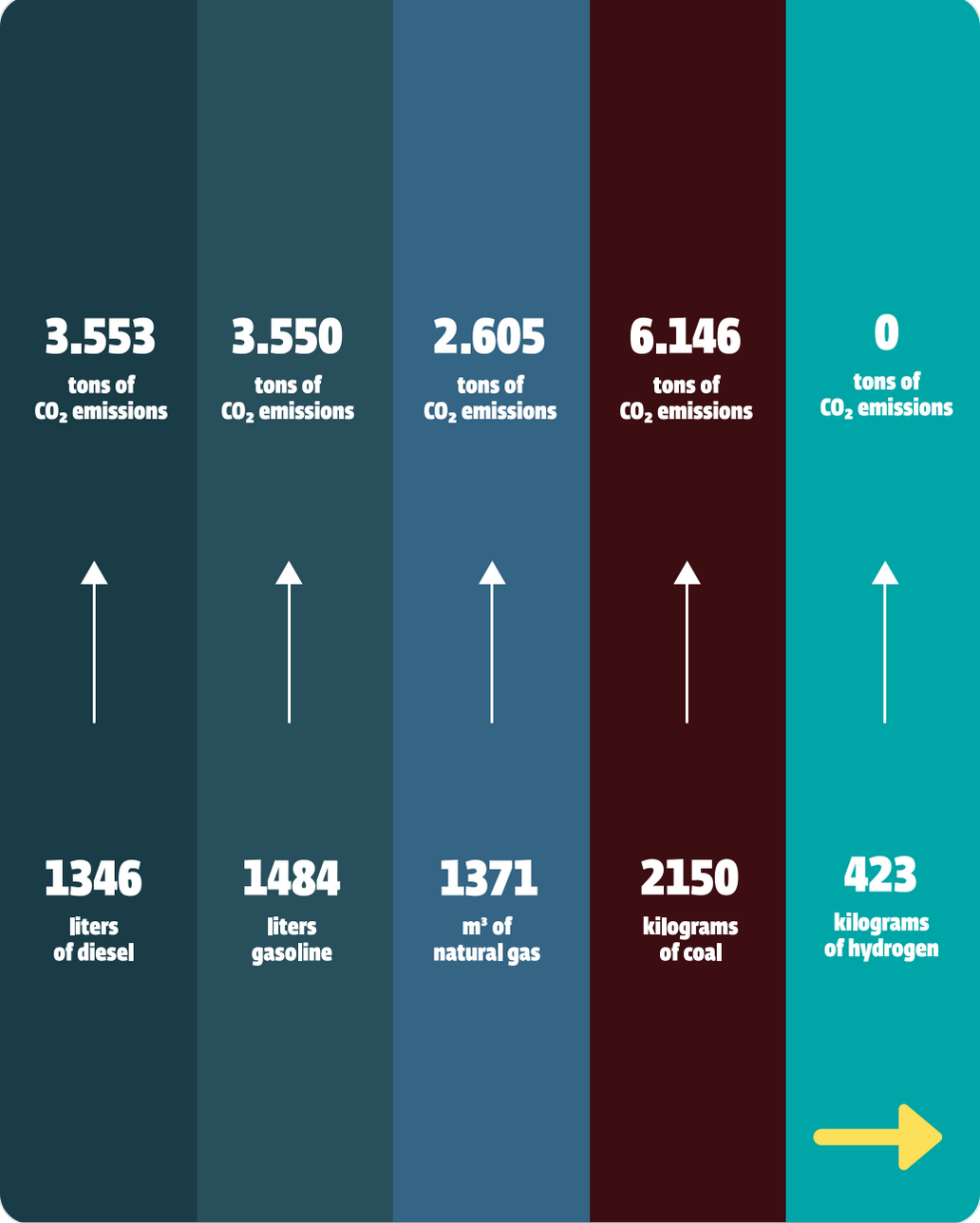
Green hydrogen generates almost zero emissions using renewable energy, making it an environmentally friendly alternative for decarbonising various sectors.
Conclusion: A Path Toward a Greener Future with Hydrogen
The environmental impacts of hydrogen production, transportation, and use depend on how the hydrogen is produced and delivered.
With its near-zero emissions, green hydrogen presents a significant opportunity to reduce global carbon footprints and advance renewable energy use.
While grey and blue hydrogen offer intermediate solutions, they still rely on fossil fuels, making them less favourable from an environmental perspective.
As countries continue to invest in green hydrogen infrastructure and technology, we are moving closer to a sustainable future where hydrogen can replace fossil fuels in industries ranging from transportation to power generation.
Contact us to learn how we can help your transition to clean energy by using green hydrogen tailored to your business needs.
With its near-zero emissions, green hydrogen presents a significant opportunity to reduce global carbon footprints and advance renewable energy use.
While grey and blue hydrogen offer intermediate solutions, they still rely on fossil fuels, making them less favourable from an environmental perspective.
As countries continue to invest in green hydrogen infrastructure and technology, we are moving closer to a sustainable future where hydrogen can replace fossil fuels in industries ranging from transportation to power generation.
Contact us to learn how we can help your transition to clean energy by using green hydrogen tailored to your business needs.
